
One Hundred Coffee is reader-supported, and some products displayed may earn us an affiliate commission. Details
Roasting coffee is more than simply applying heat to green coffee beans—it is a complex chemical process that transforms raw beans into aromatic and flavorful coffee. This transformation is driven by a series of chemical reactions, primarily the Maillard reaction and caramelization, responsible for developing the coffee’s rich flavors, aromas, and colors. In this deep dive, we will explore the different stages of roasting and the fascinating chemistry behind each phase.

Who is this for?
Perfect for aspiring home roasters and serious coffee geeks, this comprehensive guide dives deep into the techniques, science, and gear behind roasting your own beans. Learn everything from roast profiles to flavor development and troubleshooting. A must-read for those wanting to craft personalized, professional-quality coffee at home.I still remember the first time I stood next to a small drum roaster, watching pale green coffee beans tumble and slowly turn golden. The room smelled like hay at first, then hot toast, then warm caramel, and suddenly the air popped with a chorus of tiny snaps—first crack. That was the day I realized roasting isn’t just “making beans dark.” It’s an intricate, time-sensitive chemistry project that transforms dense plant seeds into an aromatic, soluble, flavor-packed food. If you’ve ever wondered what actually changes inside a coffee bean at each stage of roasting, this deep dive is for you—written from hands-on experience and grounded in the science that gives us chocolate notes, citrus sparkle, and those cozy caramel aromas we all love.
Below, we’ll walk through the roast from the moment a green bean meets heat to the final moments after second crack. We’ll connect what you see, hear, and smell to the chemical reactions happening inside the bean, then tie those changes to how your coffee will grind, extract, and taste in the cup. My goal is simple: when you pick up a bag labeled “light,” “medium,” or “dark,” you’ll know exactly what that means in molecular terms—and how to brew it beautifully.
Before the Heat: Why Green Beans Are Built for Transformation
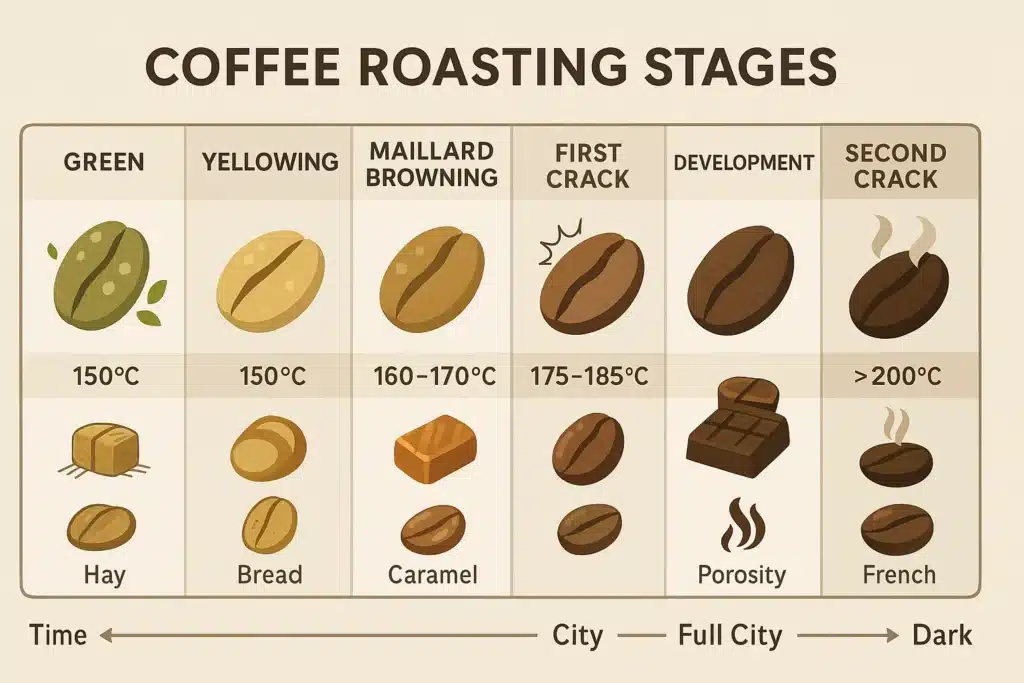
Green coffee beans are little chemical vaults. They’re dense, hard, and packed with potential—mostly water, carbohydrates (including sucrose), proteins, amino acids, organic acids (notably chlorogenic acids), lipids, and minor compounds like trigonelline. On day one, they smell a bit grassy or like dried peas. The magic requires heat to unlock flavor.
Two “pre-roast” details matter a lot:
- Water activity and moisture content. Most specialty beans hover around 10–12% moisture. That water doesn’t just evaporate—it carries heat deeper, drives pressure changes, and sets the stage for reactions. Beans with excessive moisture can roast unevenly; too low, and they may roast too quickly on the surface.
- Density and size. High-grown beans tend to be denser. Density affects how heat travels through the bean. Denser beans often handle higher charge temperatures and more aggressive heat application without scorching, while lower-density beans prefer gentler profiles.
Stage 1: The Drying Phase (Endothermic Warm-Up)

What you observe: Green beans go from pale to slightly yellowish as their surface dries. The smell shifts from grassy to something like damp straw or bread dough warming.
Chemistry under the hood: Heat energy is initially absorbed (endothermic). Water begins to evaporate, migrating from the core to the surface. This isn’t just about “getting rid of water.” The moisture gradient helps transport heat into the bean and primes the cell structure for expansion later. As internal pressure rises, microcracks begin to form—tiny pathways that will matter when we hit crack stages.
How it affects taste: Proper drying prevents “baked” flavors later. Rush this phase and you risk uneven roasting; stretch it too long and you flatten the acidity and fragrance. Think of it as warming up your pan before searing: it doesn’t create flavor by itself, but it sets up every flavor that follows.
Stage 2: Yellowing and Early Maillard Reactions
What you observe: Beans turn a uniform yellow, then light tan. The aroma veers from hay to toasted grain and sweet biscuit.
Chemistry under the hood: The famous Maillard reaction—a complex dance between reducing sugars and amino acids—begins in earnest as the bean’s temperature rises. Maillard produces a vast family of browning compounds and melanoidins, which contribute body, color, and that toasted-sweet aroma. This stage lays the scaffolding for flavor complexity. At the same time, sucrose starts to break down, and chlorogenic acids begin to isomerize and degrade, altering perceived acidity and bitterness downstream.
How it affects taste: Controlled Maillard time accentuates sweetness and complexity. Too short, and your coffee may taste green or astringent. Too long, and the cup can feel heavy and dull, with muted fruit and sparkle.
Stage 3: The Browning Phase—Aroma Explodes

What you observe: Color deepens to light brown. Aromas shift toward caramel, toasted nuts, and baking cookies. The roaster’s rate of rise (RoR)—how quickly temperature increases—becomes critical to avoid staling or racing.
Chemistry under the hood: Maillard continues, and caramelization intensifies as sucrose breaks into smaller molecules that yield caramel notes. Trigonelline begins to degrade, forming compounds including nicotinic acid (niacin) and contributing to roasted, nutty aromatics. Lipids inside the bean stay mostly intact for now, but heat is loosening the matrix that holds them, prepping for future aromatics and crema production.
How it affects taste: This is where sweetness builds. A well-paced browning phase preserves the bean’s original character (think citrus in an Ethiopian or cocoa in a Brazilian) while adding confectionery depth. Race through and you’ll get sharp acidity without sweetness; drag it and you “bake” the cup—muted, bready, tired.
Stage 4: First Crack—The Bean Becomes Porous (Exothermic Flash)
What you observe: An audible pop—like popcorn, but lighter—ripples through the drum. Beans expand rapidly; their surface may show slight chaff remnants, and the room fills with a sweet, expanded aroma. This is a turning point.
Chemistry under the hood: The bean’s internal pressure has built up enough that the cellulose structure fractures, releasing steam and CO₂. This event is exothermic—the bean briefly releases heat as it breaks. Porosity increases dramatically, which impacts solubility later when you brew. Many volatile aromatics can now escape more easily, and heat transfer characteristics change, so roaster control needs to adapt.
How it affects taste: Pull a roast shortly after first crack, and you’re likely in light roast territory—bright acidity, high origin clarity, and lighter body. Development time right after the first crack shapes the cup’s sweetness vs. sharpness balance.
Stage 5: Post–First Crack Development—Shaping Sweetness, Acidity, and Body
What you observe: Color deepens from light brown to medium. The aroma gets rounder and sweeter. You may see tiny surface flecks as chaff finishes sloughing off. Roasters talk a lot here about development time ratio (DTR)—the percentage of total roast spent after first crack—because it correlates with the balance of acidity and sweetness.
Chemistry under the hood: Maillard continues, caramelization progresses, and chlorogenic acids degrade further into compounds that can influence perceived bitterness and astringency. Lipids are mobilizing; the coffee’s oil fraction becomes more extractable later in brewing. The bean’s increasing porosity and declining density mean it will grind more easily and extract differently than a lighter roast that was dropped earlier.
How it affects taste: This is the heart of medium roasts—where you can emphasize molasses sweetness, cocoa, nuts, and gentle fruit without losing clarity. Underdevelop it (drop too soon or with too gentle a heat trajectory), and you risk a grassy, grainy cup with “peanut shell” notes. Overdo it (too much time or excessive heat), and sour-savory can tip to bitter or ashy while fruit fades.
Stage 6: Approaching Second Crack—Caramel to Carbon
What you observe: Beans darken toward deep brown. Aromas turn smoky, dark chocolate, and toasted sugar. If you keep going, you’ll hear a finer, sharper set of snaps—second crack—which signals the onset of more serious structural breakdown.
Chemistry under the hood: Caramelization has gone far; melanoidins continue to form, deepening color and body. Cellulose weakens, and oils may begin migrating toward the surface, especially beyond the second crack. While some bitterness increases, the perceived acidity drops, and the cup can shift toward roasty, smoky notes.
How it affects taste: Just shy of second crack is the classic full-city to full-city+ space—lots of caramelized sweetness and chocolate with moderate bitterness and low acidity. Push into or through the second crack and you’re in dark roast territory: bold, smoky, heavy body, and a velvety texture in espresso, but with much of the original origin character subdued.
Stage 7: Second Crack and Beyond—Pyrolysis Takes the Wheel
What you observe: The Second crack sounds finer and more rapid than the first. Surface oils often appear, and the roast color becomes very dark. Aromas turn smoky, carbonaceous, and sometimes spicy.
Chemistry under the hood: Pyrolysis—thermal decomposition without oxygen—dominates. Complex aromatic precursors have been largely driven off or converted into simple, charred compounds. Lipids migrate outward, coating the bean. PAHs (polycyclic aromatic hydrocarbons) can increase if you push very dark and smoky. On the flip side, acrylamide—which peaks earlier—declines with darker roasts.
How it affects taste: Expect low acidity, heavy body, bold roast character, and a smoother perceived bitterness if managed carefully. Very dark roasts can hide defects and deliver consistent crema in espresso, but they sacrifice origin nuance. If you love that classic “Italian dark,” this is your zone—just be mindful of freshness and storage because surface oils oxidize faster.
How Roasting Method and Airflow Change the Chemistry

Different roasters drive different dynamics:
- Drum roasters rely on conductive and convective heat. They retain heat well, which can “carry” momentum into development. Managing the rate of rise and airflow prevents scorching (burning the surface) or tipping (burning at the bean tips).
- Fluid-bed (air) roasters deliver more direct convective heat and tend to create brighter, cleaner cups with very responsive control. They can emphasize origin clarity but require careful airflow management to avoid over-drying the surface.
Airflow matters because volatile aromatic compounds are fragile. Strong airflow clears smoke and chaff, reducing smoky taints, but too much can strip aromatics or cool the system when you need thermal momentum.
The Big Three Reactions: Maillard, Caramelization, Pyrolysis
Think of these as overlapping waves rather than discrete boxes:
- Maillard is your sweetness and complexity engine. It starts early and keeps going, producing melanoidins that influence the body and color.
- Caramelization converts sucrose into caramel and toffee-like compounds. It intensifies from mid-browning through early development.
- Pyrolysis dominates late. It creates roasty, smoky characteristics, can produce surface oils, and will eventually carbonize if pushed hard.
Balancing those waves lets you tune for lemon-honey brightness, cocoa-caramel richness, or smoky-dark boldness.
What Happens to Specific Compounds (And Why You Taste Them)
- Chlorogenic acids (CGAs): Abundant in green coffee; they degrade with heat. Light roasts retain more CGAs, which contribute brisk acidity and sometimes astringency. Medium roasts often taste sweeter because CGA breakdown products balance acidity with cocoa-like bitterness.
- Sucrose: Declines steadily with heat; caramelization products bring caramel/toffee sweetness and mellow acidity, peaking around medium roasts before giving way to darker, roast-dominant notes.
- Trigonelline: Degrades into nicotinic acid and other compounds that layer roasted, nutty notes and a subtle bitter edge.
- Lipids: Largely preserved internally until dark roasts, where migration to the surface can boost crema in espresso but also accelerates rancidity if storage is poor.
- Proteins and amino acids: Feed Maillard; their reactions build the brown color and umami-like depth.
Roast Color, Agtron Numbers, and Why Color Isn’t Everything
Roasters often reference Agtron numbers (a color scale) to communicate roast degree. Light might sit in the high 70s/80s, medium in the 60s, dark in the 40s/50s. Color is useful, but it’s only a proxy for chemistry. Two beans can share the same external color but taste different if one spent longer in the Maillard or had a different post-crack development path. That’s why experienced roasters also track the rate of rise, gas/airflow changes, and time from first crack to dial in flavor.
Defects You Can Taste: Scorching, Tipping, Baking, Underdevelopment
- Scorching & tipping: Surface burns from excessive conductive heat or too-hot charge temps. Tastes ashy, bitter, sometimes like burnt toast.
- Baking: A too-long, too-flat roast, often from a collapsing RoR. Tastes bready, papery, muted, with low sweetness and no sparkle.
- Underdevelopment: Dropping too early or starving the roast of sufficient energy. Tastes grassy, peanut-shell, sharp astringency with hollow sweetness.
Each defect is a roast-chemistry mismatch: either heat didn’t move the reactions along cleanly, or it moved too aggressively in the wrong way.
Degassing and Resting: The Quiet Chemistry After Roasting

Freshly roasted coffee emits CO₂ as a byproduct of roasting. This degassing affects extraction and flavor clarity. Light roasts often need more rest (sometimes 5–10 days) to stabilize; darker roasts degas faster and might peak after 2–4 days. Espresso, in particular, benefits from more rest to tame gassy, uneven shots. During resting, volatiles redistribute, and the cup often sweetens and clarifies.
How Roast Level Changes Your Brew
Roast chemistry dictates how you should grind and extract:
- Light roasts are denser and less soluble. Use finer grinds, higher water temps (94–96°C), and longer contact. Expect bright acidity, florals, and fruit. Under-extraction shows up as harshness; push your extraction a bit more than you would for a medium roast.
- Medium roasts are crowd-pleasers. They’re more soluble, allowing a wider brew window. Balanced acidity and sweetness make them forgiving. Brew around 92–95°C with a grind appropriate to your method.
- Dark roasts are highly soluble. Use slightly coarser grinds or lower temperatures (88–92°C) to avoid over-extraction and bitterness. Expect low acidity, heavy body, chocolate-smoke notes, and thick crema in espresso.
With espresso specifically, roast chemistry shows up in flow behavior. Darker roasts often flow faster at the same grind setting due to greater porosity and surface oils. Adjust your grind and dose to target a sweet, syrupy extraction rather than a thin gusher.
Origin vs. Roast: Who Wears the Crown?
Roast doesn’t replace origin—it interprets it. Light roasts showcase terroir: berries in natural Ethiopians, citrus in washed Kenyans, and cocoa-nut in Brazils. Medium roasts harmonize terroir with caramelized sweetness. Dark roasts elevate roast-driven flavors and texture, which can be wonderful in milk drinks and classic espresso. The “right” roast is the one that aligns with how you brew and what you crave.
Safety, Freshness, and Storage
A quick word on safety chemistry: acrylamide forms early in roasting and typically declines with darker roasts; PAHs can increase with very dark, smoky roasts. For freshness, oxygen is the enemy. Store beans in a valve bag or an airtight canister, away from heat and light. Surface oils on dark roasts oxidize faster, so enjoy those bags sooner. Grind right before brewing to preserve volatile aromas—your nose and cup will thank you.
Tasting the Stages: How to Learn Roast Chemistry With Your Senses
If you want to feel the chemistry, do a side-by-side of the same coffee roasted light, medium, and dark. Smell each dry, then after grinding, and then when blooming with hot water. Notice how light roast carries floral and fruit notes that leap out, medium roast smells sweet and cocoa-like, and dark roast leans smoky and chocolate. On your tongue, follow the acidity-sweetness-bitterness arc from bright to balanced to bold as roast deepens. That arc is Maillard, caramelization, and pyrolysis expressed as flavor.
Troubleshooting Flavor: What the Cup Is Trying to Tell You
- Too sour, sharp, or thin? You might be brewing a light roast too cool or too coarse. Increase temperature, grind finer, or lengthen contact time. If the roast itself is underdeveloped, no brew trick will fully fix the grassy edge, but a slightly longer extraction can help.
- Muted, bready, flat sweetness? That can signal a baked roast or an overly slow brew. Try a finer grind and a slightly higher temperature; if it’s the roast, choose a different roaster or profile next time.
- Bitter, ashy, smoky? That’s classic dark-roast over-extraction or a roast pushed too far. Coarsen the grind, shorten the contact time, or drop brew temp a degree or two.
Why Professional Roasters Watch the Rate of Rise (RoR)
RoR charts show how quickly bean temperature climbs. A smoothly declining RoR—fast early, gentler later—helps prevent stalled browning (baking) and surface burns (scorching). The chemistry is time-temperature dependent: you want enough momentum to carry Maillard forward without bulldozing into pyrolysis too soon. Think of RoR as your steering wheel for flavor.
Beyond the Drum: Profile Styles and Flavor Goals
- Scandinavian-style light roasting often emphasizes short overall times with a lively Maillard and a gentle, precise development. Expect lifted florals, citrus, and a crisp finish.
- Classic specialty medium stretches Maillard for rounded sweetness, then gives a clean, controlled development for chocolate, nougat, and balanced fruit.
- Traditional dark espresso rides development longer, courting surface oils for a syrupy body and a velvet crema, designed to punch through milk while remaining smooth.
Each style is a different chemistry map across the same landscape of compounds.
The Best Part: Let Roast Chemistry Guide Your Brewing Choices
Once you understand where a coffee sits on the chemistry curve, you can dial your grinder, water temperature, and brew ratio like a pro. Light roast with lively acidity? Give it heat and time. Medium roast with caramelized sweetness? Aim for balance and clarity. Dark roast with oily sheen? Reduce heat and avoid over-extraction to keep it velvety and bold.
Light, Medium, Dark—A Chemistry-First Summary

- Light roast: Higher CGAs, more trigonelline left, less caramelization and pyrolysis. Expect high acidity, origin clarity, and floral/fruit aromatics. Needs a finer grind and hotter water for proper extraction.
- Medium roast: Balanced Maillard and caramelization, moderated CGAs, growing melanoidins. Expect sweetness, cocoa/nut, rounded fruit, balanced acidity. Versatile across brew methods.
- Dark roast: Caramelization gives way to pyrolysis; surface oils are often present. Expect low acidity, heavier body, smoky chocolate, with origin nuances subdued. Use slightly cooler water and a coarser grind to avoid bitterness.
From Roaster to Ritual: Bringing It Home
Here’s my favorite way to “read” a new bag using the chemistry you just learned:
I start with a balanced pour-over recipe (say, 1:16) at 94–95°C for a medium roast, taste, then adjust. If the bag is light, I increase the temperature to 96°C and grind a touch finer to coax sweetness and tame edgy acidity. If it’s dark, I step down to 90–92°C and grind a notch coarser to avoid bitterness while highlighting body and chocolate. I taste on days two, four, and seven to catch the degassing curve. Almost always, the cup is sweetest and most complete a few days post-roast.
For espresso, I watch how the shot flows more than the timer at first. Light roasts often demand a finer grind and higher temperatures; dark roasts flow faster and benefit from a slightly coarser grind and a cooler group temp if you can manage it. The colors of the stream tell you when you’re balancing acidity, sweetness, and roast bitterness—again, chemistry made visible.
Final Sips: Why Understanding Roast Chemistry Makes Coffee More Fun
Coffee roasting is a symphony of drying, Maillard browning, caramelization, and pyrolysis, conducted in minutes and measured in aromas, colors, and delightful cracks. Knowing what’s happening inside the bean helps you buy more confidently, brew more precisely, and troubleshoot with intent. It’s also simply more fun. That first whiff of biscuit in yellowing, the gentle crescendo to first crack, and the deep chocolate glow of a well-paced development stage—these aren’t random moments. They’re predictable, repeatable markers of flavor chemistry unfolding.
Whether you’re a curious home brewer, a budding roaster, or just someone who loves an excellent cup, the journey from green seed to aromatic bean is now an open book. You can choose a light roast for the sparkle of origin, a medium for caramel-cocoa balance, or a dark for plush, smoky comfort—and brew it in a way that honors the chemistry that put those flavors there. That’s the real reward: your daily coffee becomes not only tastier, but also more understood—and once you understand it, you can make it yours.






